CALIBRE™ MEGARAD™ 2091-15
| 牌号 | CALIBRE™ MEGARAD™ 2091-15 |
| 厂家 | Trinseo |
| 分类 | PC |
| 介绍 | CALIBRE™ MEGARAD™ 2091 Polycarbonate Resins are designed for single use medical devices (SUDs) that require gamma and electron-beam irradiation and an accelerated schedule for delivery of post-radiated products to customers - potentially 10 to 21 days sooner than currently available radiation-stabilized polycarbonate resins, depending on irradiation conditions. In addition, the resins are meant for applications that require a more water white appearance rather than the traditional purple tinted resin used to compensate for these sterilization methods. CALIBRE™ MEGARAD™ 2091-15 Polycarbonate Resin has undergone biocompatibility testing based on ISO 10993 standards (Biological Evaluation of Medical Devices) and is suitable for use in approved medical applications. Main Characteristics
Applications
|
| Trinseo 产品展示 | 种类 |
|---|---|
| EMERGE™ PC 8600-20 | PC |
| CALIBRE™ 200-3 | PC |
| EMERGE™ PC 4350 | PC |
| EMERGE™ PC 8430-22 | PC |
| CALIBRE™ 302-6 | PC |
| CALIBRE™ 302V-6 LD | PC |
| CALIBRE™ 502-3 | PC |
| CALIBRE™ 201-18 | PC |
| CELEX™ 310HF.M | PC |
| CALIBRE™ 301-58 LT | PC |
订购说明
上海松翰塑化科技有限公司订货流程
【订购导引】
我司所销售所有产品均为原厂原包,均可提供相关资料报告(如:物性表,MSDS报告,UL报告,COA报告,SGS报告,FDA等)!
如您需要提前了解原料产品的相关性能或是需要原料产品的相关报告,请您通过电话或是客服QQ与我联系!
【物流说明】
自接到贵司订购单24小时内发货,珠三角,长三角,北京,天津均支持代收货款。货物送达后,请第一时间检查外包装,如发现物流过程中出现大量包装损坏及原料外漏的,请让物流人员出具证明并保存,并在24小时内与我司客服部取得联系。
【订购流程】
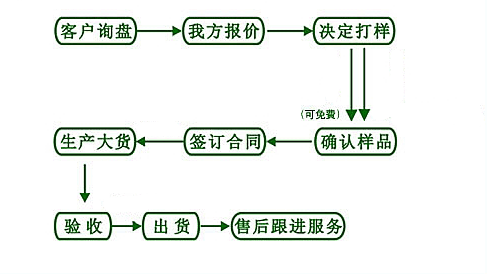
【退换货处理】
退货:
依照中华人民共和国消费者保护法规,在您收到货品7天内,若发现您收到的商品有瑕疵或损坏,您可以要求退货,请主动与本公
司联络,敝公司接到您的商品之后,会将您的购物金额全额退回。
换货:
若您收到的商品有瑕疵或损坏,请您于7天内与本公司联络,本公司有专人处理换货事宜。
我司所销售所有产品均为原厂原包,均可提供相关资料报告(如:物性表,MSDS报告,UL报告,COA报告,SGS报告,FDA等)!
如您需要提前了解原料产品的相关性能或是需要原料产品的相关报告,请您通过电话或是客服QQ与我联系!
【物流说明】
自接到贵司订购单24小时内发货,珠三角,长三角,北京,天津均支持代收货款。货物送达后,请第一时间检查外包装,如发现物流过程中出现大量包装损坏及原料外漏的,请让物流人员出具证明并保存,并在24小时内与我司客服部取得联系。
【订购流程】

【退换货处理】
退货:
依照中华人民共和国消费者保护法规,在您收到货品7天内,若发现您收到的商品有瑕疵或损坏,您可以要求退货,请主动与本公
司联络,敝公司接到您的商品之后,会将您的购物金额全额退回。
换货:
若您收到的商品有瑕疵或损坏,请您于7天内与本公司联络,本公司有专人处理换货事宜。


